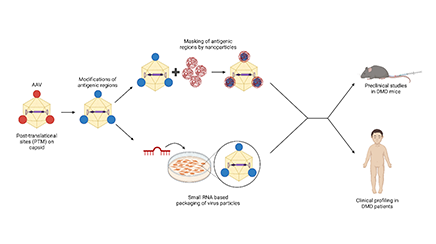
Duchenne Muscular Dystrophy (DMD), is a severe neuromuscular disorder in humans (1:3500 male births). DMD leads to progressive muscle wasting in affected boys, who ultimately succumb to the disease due to heart or lung failure. Gene therapy, a method to replace the altered gene (dystrophin) with a normal copy, has not been very effective for this condition.
The project proposes to address this, first by designing AAV9 vectors with improved transduction and immune evasive potential. A combination of rational engineering of viral capsids strategically modified at the rate limiting post translational modification (PTM) sites, and which overlap with antigenic epitopes, combined with nanoparticle mediated epitope masking is likely to overcome these major barriers in DMD gene therapy. Further optimization such as the microRNA-based vector production, will further complement and enhance the functionality of AAV9 vectors. Finally, in preparation for a possible clinical translation, we will also profile the genetic alterations and immune status in a large number of patients with DMD to provide clinical insights on the role of recipient status in future gene therapy trials.