
M. L. N. Rao
PhD (University of Hyderabad)
Professor, Department of Chemistry
CL 204E/ Core Labs ,
Department of Chemistry
IIT Kanpur,
Kanpur 208016
विशेषज्ञता
Organometallics for organic synthesis
शिक्षा
PhD (1996), University of Hyderabad
M. Phil. (1990), University of Hyderabad
M. Sc. (1988, Org. Chem.), Nagarjuna University
चयनित प्रकाशन
पुरस्कार एवं फैलोशिप
पेशेवर अनुभव
IIT Kanpur, 2003-
University of North Carolina (UNC) Chapel Hill, USA, 2002-2003
Post Doc at NIMC, NIAR and AIST, Tsukuba, JAPAN, 1997-2002
वर्तमान शोध
Our primary research revolves around the 'development of green organometallic reagents and their applications to organic synthesis'. We embarked upon the development of 'new generation cross-coupling reactions' using triarylbismuths as 3-fold coupling reagents. Over the years, we have developed a variety of new coupling reactions using organobismuth chemistry, with a diverse range of reactivity under palladium catalyzed conditions with high atom-economy. Further research activities in our group include convergent organic synthesis, microwave mediated organic synthesis, auto-catalysis, metal catalyzed reactions and other reactions of contemporary interest.
New Generation Green Cross-Coupling Reactions: Cross-coupling methods have enriched the art of organic synthesis and evolved as effective synthetic tools to construct complex molecular systems. The well-known coupling reactions such as Suzuki, Stille, Negeshi, etc. invariably involve only one C-C bond formation (eq. 1).
![]() We envisaged that a paradigm shift in the reagent capability is necessary to make these reactions more green and atom-economic with additional potential for multi C-C couplings in one-pot operation.
We envisaged that a paradigm shift in the reagent capability is necessary to make these reactions more green and atom-economic with additional potential for multi C-C couplings in one-pot operation.
Triarylbismuths, which are non-toxic, air stable, and contain three aryl groups (e.g., Figure 1), appeared to us as the most promising green organometallic reagents with 3-fold coupling capabilities (eq.2).
 These reagents could, in principle, be employed in sub-stoichiometric 1/3 molar equivalents with increased atom-efficiency in a one-pot operation (eq. 2 vs eq. 1). Our consistent efforts have led to the development of new generation cross-coupling reactions with triarylbismuths reagents, and opened up a plethora of new opportunities in terms of reactivity and selectivity. Thus, a new series of Pd-catalyzed coupling reactions have been developed involving aryl, heteroaryl, acyl, allyl and vinyl couplings, bis-couplings, carbonylations and domino one-pot coupling reactions.
These reagents could, in principle, be employed in sub-stoichiometric 1/3 molar equivalents with increased atom-efficiency in a one-pot operation (eq. 2 vs eq. 1). Our consistent efforts have led to the development of new generation cross-coupling reactions with triarylbismuths reagents, and opened up a plethora of new opportunities in terms of reactivity and selectivity. Thus, a new series of Pd-catalyzed coupling reactions have been developed involving aryl, heteroaryl, acyl, allyl and vinyl couplings, bis-couplings, carbonylations and domino one-pot coupling reactions.
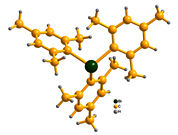 Figure 1.The Structure of trimesityl-bismuthine
Figure 1.The Structure of trimesityl-bismuthine



