PI: Prof. Sankar Prasad Rath
This project is aimed at biomimetic study of di/multi-heme proteins in order to understand the structure-function relationships at the molecular level. The presence of more than one redox center provides the Nature with a further tool to modulate various properties via heme-heme interactions that can be cooperative or anti-cooperative but both having functional consequences. Active site analogues can explain various aspects of Nature's sophisticated design to develop such architectures. Model of the di/multi-heme centers will be synthesized in which two or more porphyrin macrocycles are covalently bound by spacers. Judicious choice of the spacer will allow precise control in the spatial arrangement for inter-macrocycle interactions and possible electronic communications. Focus will be on how the nature and extent of heme-heme interactions influence the structure, function and properties of the individual heme centers.
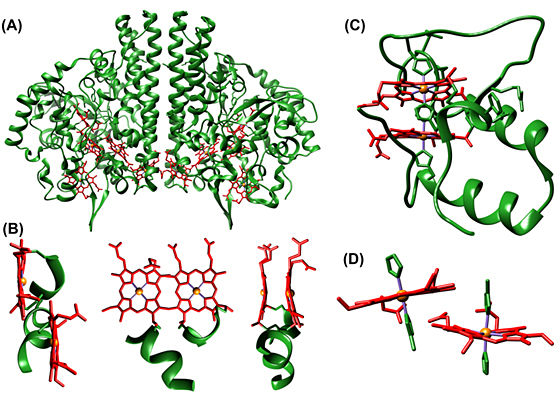
Figure:
(A) Cytochrome c nitrite reductase (PDB code 1QDB) and
(B) diheme motifs therein.
(C) Di-heme cytochrome c2 (PDB code 2CZS) and
(D) without protein chains.